Chemical characterization refers to the identification, qualitative, and quantitative analysis of chemicals present in materials or finished medical devices.
※ Qualitative analysis of materials used in medical device manufacturing |
※ Characterization of manufacturing materials through qualitative and quantitative chemical composition analysis |
※ Characterization of chemicals introduced during the manufacturing process (such as processing aids, process contaminants, sterilization residues) |
※ Estimation of the potential release of chemicals from medical devices or their manufacturing materials under clinical use conditions |
※ Measurement of chemicals released by medical devices under clinical use conditions |
01 Physicochemical Testing
Clarity, color, chlorides, pH, total heavy metals content, heavy metal elements analysis, UV absorbance, reducing substances (oxidizable substances), ammonium, ash on ignition, residue on evaporation, ethylene oxide residue, 2-chloroethanol residue, vinyl chloride monomer residue, solvent residues, moisture content, molecular weight and molecular weight distribution (GPC), osmotic pressure molarity
Laser particle size analyzer, UV-Vis spectrophotometer, elemental analyzer, polarimeter, direct reading spectrometer, rotational viscometer
Method Reference: ISO 10993-18 / GB/T 16886.18]
Category | Analytical Method |
Spectroscopic Analysis | FTIR, EDXRF, WDXRF, XRD and ICP |
Chromatographic Testing | GC, HPLC, GPC, IC |
Mass Spectrometry | MS, ICP-MS, GC-MS(MS), LC-MS (MS), Q-TOF, PY-GC-MS, HS-GC-MS, Q ExactiveTMHF |
Thermal Analysis | DSC, TGA, DMA, DSC-TGA |
NMR Analysis | H spectrum, C spectrum, P spectrum, F spectrum, DEPT spectrum, and two-dimensional spectra (HMQC, HSBC, COSY, NOSY, etc.) |
Other Tests | Laser particle size analyzer, UV-Visible spectrophotometer, elemental analyzer, polarimeter, direct reading spectrometer, rotational viscometer. |
Method Reference: ISO 10993-18 / GB/T 16886.18
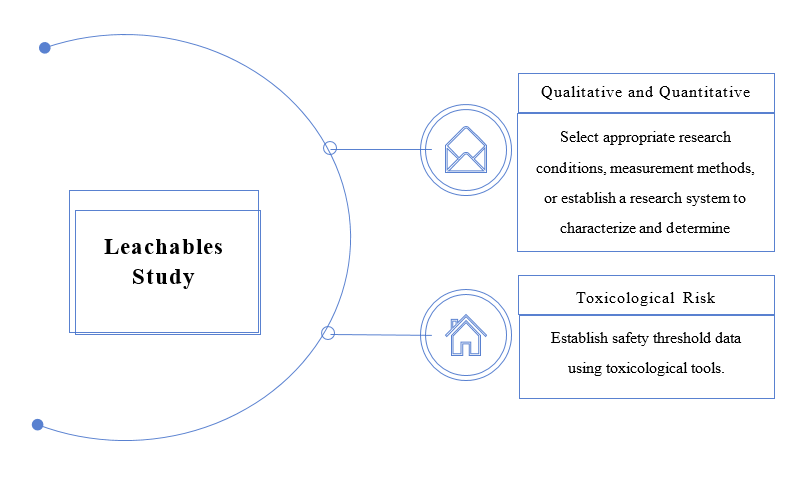
02 Leachables Study
Leachables are chemical substances released from medical devices during their continuous contact with the human body and functioning, or during interaction with other media used in application (such as blood, medicinal solutions, etc.). Leachables are categorized into two types: known leachables (Target Leachables), which are identified based on relevant information, and unknown leachables, identified through a research system for unknown substances.
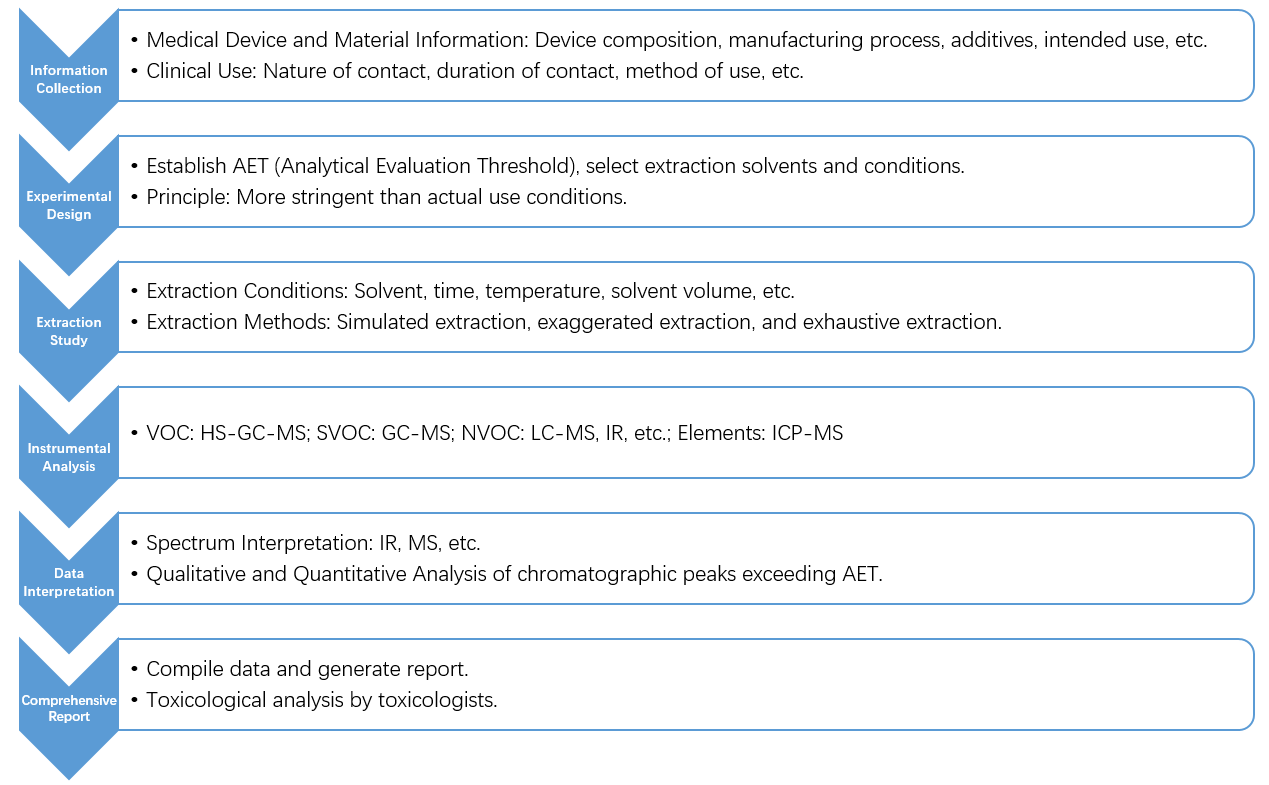
Unknown Leachables Service Process
CIRS Testing focuses on providing precise material chemical characterization services for the medical device industry, ensuring that each product complies with strict medical standards in terms of material safety and performance. From raw material analysis to final product chemical safety assessment, CIRS Testing offers comprehensive and detailed testing solutions to help you effectively address various challenges in clinical applications.
Our Advantages
Whole Industry Chain Services|Provide full industry chain technical services, including R&D support, safety evaluation and testing, registration, clinical, quality management system, etc.
Professional Technical Team | A team of experts in the fields of medical device regulations, registration, medicine, toxicology, statistics, biological evaluation, chemical analysis, microorganisms, etc., with comprehensive technical service capabilities
Professional Laboratory|Own several professional laboratories, including chemical analysis lab, materials lab, microorganisms lab, environment lab, efficacy evaluation lab, animal safety evaluation lab, etc.
Rich Industry Experience|Successful cases on safety assessment, registration and clinical services in active, non- active and IVD; Rich experience in implantable degradable products, medical beauty products, oral materials, cavity stents, assisted reproductive products and orthopedic ophthalmology field.
If you have any needs or questions, please contact us at test@cirs-group.com.
